By Jemimah Wellington, JKNMedia Reporter
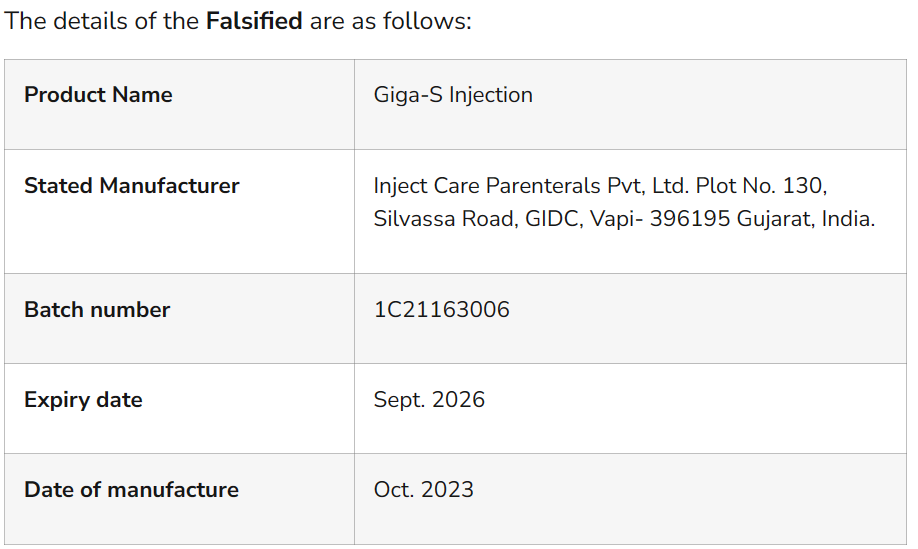
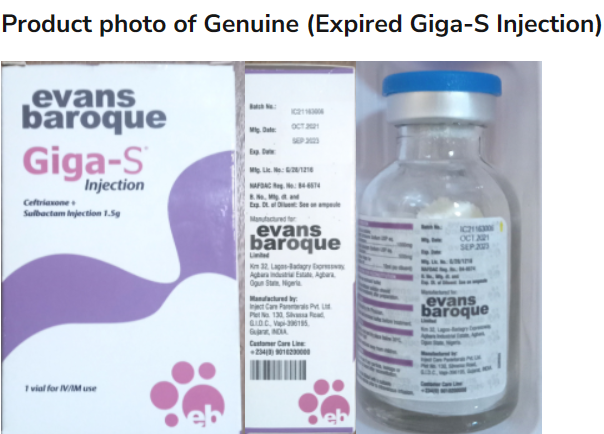
A COUNTERFEIT batch of Giga-S Injection has been identified in Aba, Abia State, prompting a nationwide alert from the National Agency for Food and Drugs Administration and Control (NAFDAC).
NAFDAC notes that the falsified product, purchased from a well-known establishment in Araria Market, Aba, does not meet the standards of the authentic Giga-S Injection, which is used to treat severe infections such as septicemia, pneumonia, and meningitis.
The agency notes that while the falsified batch was discovered in Abia State, there is concern that it may have already entered other regions of the country through informal markets, according to the issued Public Alert No. 019/2024 – Alert on falsification of a batch of Giga-S Injection and posted on its X/Twitter page.
Originally, Giga–S injection Ceftriaxone + Sulbactam Inj.1.5g IM/IV is indicated for the treatment of severe infections due to susceptible organisms including septicemia; pneumonia; and meningitis, the NAFDAC states.
NAFDAC then urges caution among importers, distributors, retailers, and healthcare providers to ensure that all medicinal products are sourced from authorized suppliers and thoroughly verified for authenticity and quality.
It also advises individuals in possession of the affected product to cease its use and promptly surrender it to the nearest NAFDAC office.
Healthcare professionals are also encouraged to report any cases of suspected falsified medicines to NAFDAC via dedicated channels while consumers who have used the counterfeit product and experienced adverse reactions are urged to seek immediate medical attention.
NAFDAC also urges that for further information, concerns should be reported to its contact line 0800-162-3322 or via email at sf.alert@nafdac.gov.ng.
Additionally, adverse events related to medicinal products can be reported through the NAFDAC website or the Med-safety application as this notification will also be shared with the WHO Global Surveillance and Monitoring System to enhance global awareness and monitoring efforts.

NAFDAC says it remains committed to safeguarding public health through rigorous regulatory oversight and proactive consumer protection measures.





